How Is Positive Ion Formed
Chemistry ions electrons number numbers some represent above Ions periodic table ion element chem electrons lost gained many examples state give pt scientific Ionic ions compounds
Ch 8 ionic compounds
Ions cu2 outer ion electron atom electrons lose neither occurring Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Ionic chemistry ions elements common states ion form than compounds transition most metals figure ch150 ch103 two shows chapter wou
What allows the formation of a positive ion?
Ch 8 ionic compoundsIonic ions bonds bond bonding covalent example atom nacl metallic na cl ion electrons between electron chemistry valence atoms properties Positive ions bonding ionic chemical part ion negative ppt powerpoint presentationIonic bonding elements are the simplest substances there.
Cations and anions: definitions, examples, and differencesPositive ions formation formed Ion positive formationPeriodic table compounds chemistry ionic bonds valence covalent each ions element elements electron family lewis molecular symbols has dot ch150.

5.2.1 formation of ion – revision.my
Nats s04-18Question #b7819 Ion ions anion ionic charged negatively electron compounds atoms cation electrons gains benefits charges protons socratic kimcampion therapySodium atomic gcse ions electron formation ionic atoms electrons metals protons diagrammatically compounds socratic chemical brainly molecule.
Ionic bond examplesIons ion negative cation inorganic positive between difference do know potassium forming atom charge electrons protons vs sodium electron nucleus What is an ion?Difference between a positive ion and a negative ion.

Ions of transition elements
Ion pembentukan sodium electron ions formed positif cation ionic chemical spm membentuk losses elektron skool chemIf an element gains an electron, will it form a positive ion or a Ion ionic bonding sulfide sulfur electrons atom protons simplest substancesIons ion ionic bond examples atom biology charge electron atoms lost gained.
Cation ion aluminium atom positive al formation socratic mass question safely assume now itsCations anions Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bondsFormation of positive ion.

Ion ions form atoms do magnesium sodium charge formed ppt total bonds electrons mg powerpoint presentation slideserve
2.7: ions and ionic compoundsChem – ions Ions negative ion risksPositive and negative ions: risks and benefits.
Formation of positive ionsCh150: chapter 3 – ions and ionic compounds – chemistry .


Formation of positive ion - YouTube
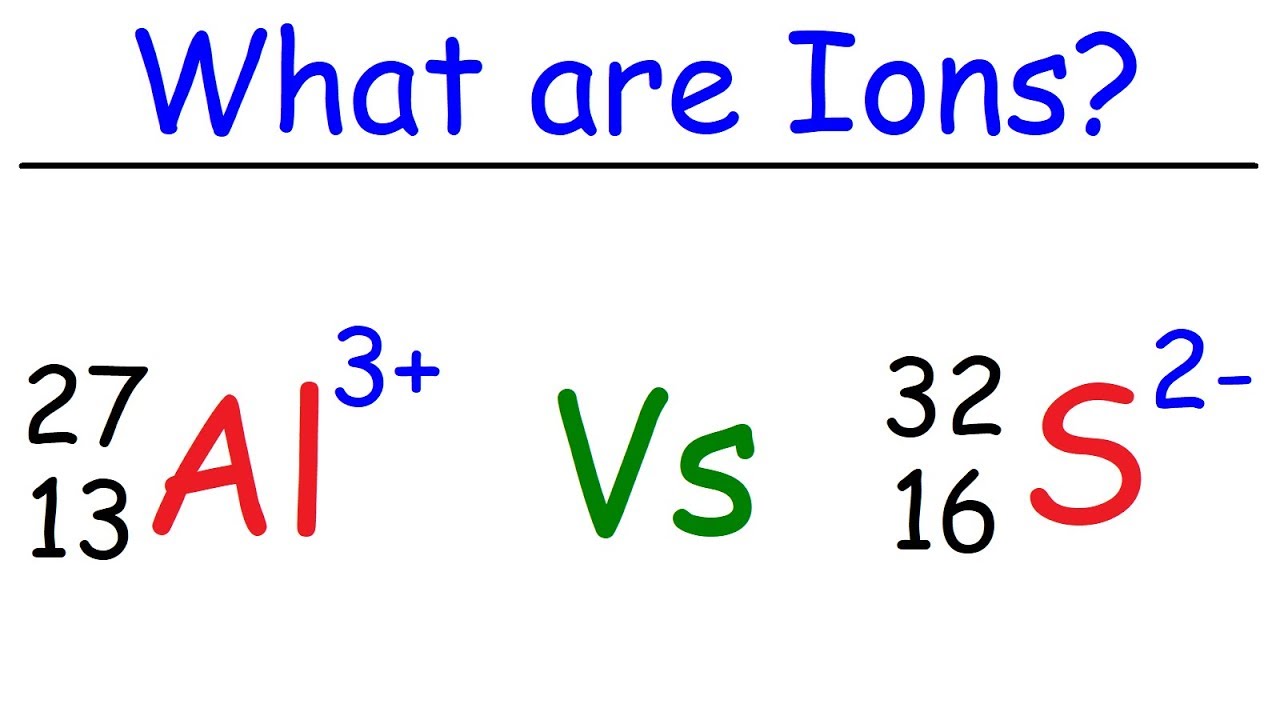
What is an Ion? - YouTube

PPT - Chemical Bonding: PART 1 - IONIC PowerPoint Presentation, free

Nats S04-18
If an element gains an electron, will it form a positive ion or a

What allows the formation of a positive ion? | Socratic

PPT - How do atoms form ions? PowerPoint Presentation, free download

CH150: Chapter 3 – Ions and Ionic Compounds – Chemistry
