How Many Protons Electrons And Neutrons In Na
Isotopes: definition, explanation, properties and examples Electrons protons neutrons atom slidetodoc atoms Hollyaschemblog: 9/21/15 in class
Using The Periodic Table To Determine Protons Neutrons And Electrons
Na+symbols+find+the+1++number+of+protons+number+of+neutrons – dynamic Proton, electron, neutron The number of protons, neutrons and electrons
Periodic protons neutrons table electrons elements chemical atoms ph polar determine using glucose bu covalent basic living matter chemicals these
Proton neutron electron atom definition chemistry particles three formula worksheet applicationProtons neutrons electrons atom periodic lithium Periodic table of elements list with protons neutrons and electronsHow to find the number of protons, electrons, neutrons for helium (he.
Protons neutrons sodium atomic periodic electron periodictableUsing the periodic table to determine protons neutrons and electrons Protons neutrons electrons atom periodic atomic rounded powerpoint img30 pte buffalostate wileyProtons neutrons electrons number helium find he.

Protons neutrons electrons number
Boron atom atomic google protons electrons neutrons number mass find model science atoms search chemie van diagram which calculation averageHow many protons neutrons and electrons does gold have Atoms and the periodic tableNeutrons neutron nucleus atomic protons electrons particles isotopes orbital atoms subatomic made.
Protons xenon neutrons electrons electron atomic proton nucleusNeutrons protons electrons number many nuclide notation atoms isotopes ions periodic atomic table proton mass neutron electron element numbers same Neutrons protons electrons number many nuclide notation isotopes atoms atomic mass proton neutron electron element ions numbers elements same periodic.


Using The Periodic Table To Determine Protons Neutrons And Electrons

The Number of Protons, Neutrons and Electrons - YouTube
.PNG)
Isotopes.
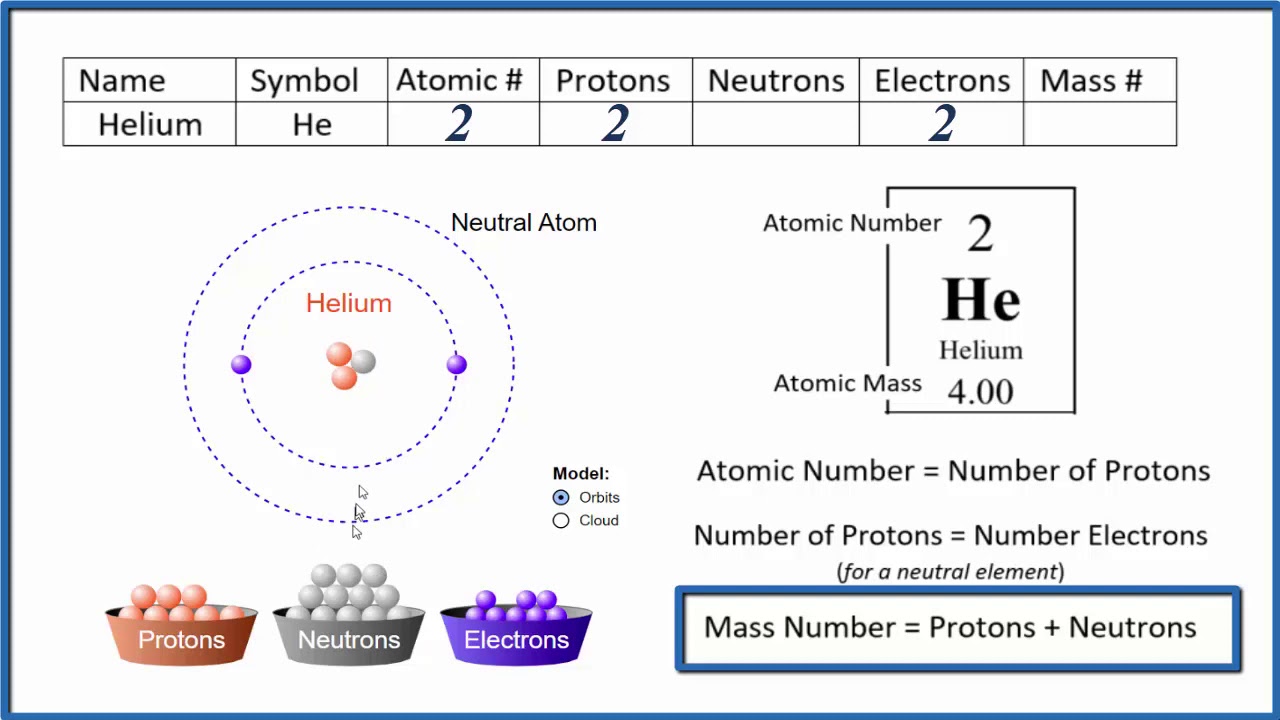
How to find the Number of Protons, Electrons, Neutrons for Helium (He

Na+Symbols+Find+the+1++number+of+protons+number+of+neutrons – Dynamic

Proton, Electron, Neutron - Definition - Formula - Application

Isotopes: Definition, Explanation, Properties And Examples

PPT - How many protons , electrons, and neutrons are in an atom

How Many Protons Neutrons And Electrons Does Gold Have - Shedding Light
.PNG)
Atoms and the Periodic table - Presentation Chemistry
